Plant-based production of glyco-engineered nematode vaccines
Parasitic nematodes are worldwide amongst the most common pathogens in grazing ruminants. The continuous exposure to these worms has a significant impact on the health status and productivity of the animals. Control of these infections currently relies almost completely on periodic mass administration of anthelmintic drugs. However, with the increasing incidence of anthelmintic resistance around the world, there is an urgent need for alternative control measures.
Vaccination is often put forward as the most rational and cost-effective alternative to control infections with parasitic worms. The induction of a protective immune response following vaccination with recombinantly produced worm antigens has shown to be a major hurdle in the development of vaccines against worms. The reason for failure has often been attributed to inappropriate recombinant expression and in particular the lack of their native post-translational glycan modifications. In recent years significant progress has been made on adapting the post-translational machinery of plants, such as Nicotiana benthamiana, to synthesise vaccine proteins with a defined and tailored glycan composition.
The aim of this project is to use this versatile plant-based production platform to express a set of well-defined nematode vaccine antigens and deliver proof-of-concept on the role of the glycans on vaccine efficacy against nematodes.
The research workflow designed to achieve the goals set forward is schematically depicted in the figure below. The workflow is designed to produce glycan-engineered ASP-based anti-nematode vaccines and test their immunostimulatory and protective capacities in sheep and cattle.
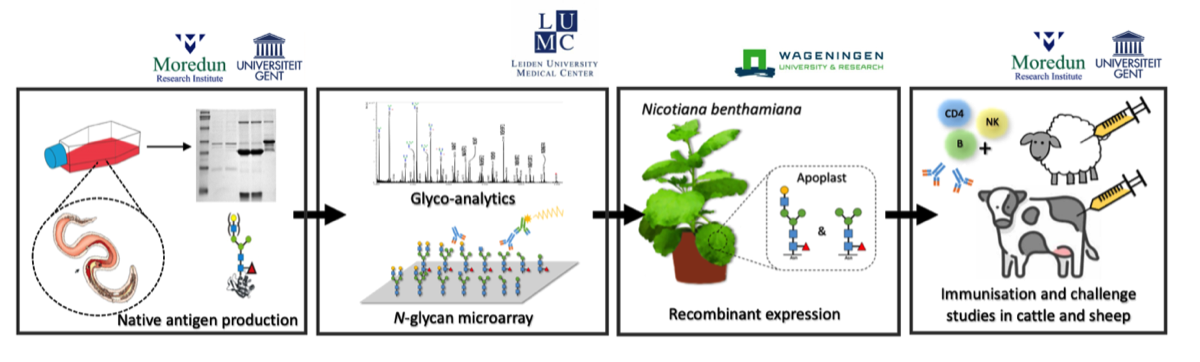
Impact & relevance
The induction of a protective response following vaccination with recombinantly produced worm antigens has shown to be a major hurdle in the development of vaccines against worms. The reason for failure has often been attributed to inappropriate recombinant expression. However, for most of the antigens evaluated in the past there was actually no scientific proof that the native proteins themselves had any protective capacity. As a matter of fact, there are only a handful of native worm proteins that have been evaluated in vaccine studies. Without any information on the type of immune response needed or the protein and/or glycan epitopes involved in such response, it has been impossible to make any informed decisions concerning the recombinant expression strategy. By comparing the vaccine induced immune responses between the protective (native) and non-protective (recombinant) versions of the ASP-based vaccines, together with detailed biochemical and glycoanalytical work, we wish to investigate whether the N-linked glycans are indeed involved in the vaccine induced immune response. Furthermore, it has now also become technically possible to reconstruct these natural glycans. Combining this expertise could provide a major breakthrough in parasite vaccine development.
Project management and responsibilities:
The project is a joint effort of four research labs from Belgium, the Netherlands and the UK.
Prof. Dr. Peter Geldhof – Ghent University - Belgium
Expertise: parasitology and vaccinology
Dr. Alasdair Nisbet – Moredun Research Institute - UK
Expertise: parasitology and vaccinology
Dr. Ron Hokke – Leiden University Medical Center – The Netherlands
Expertise: helminth glycomic and host-parasite glycobiology
Dr. Ruud Wilbers – Wageningen University – The Netherlands
Expertise: plant-based expression and glycan-engineering.